Overview of LiAl-LDHs Sorbents Used in Direct Lithium Extraction.
As lithium demand grows, projects have been adapting a decades-old DLE medium to transition from bench and pilot scale to commercial production.
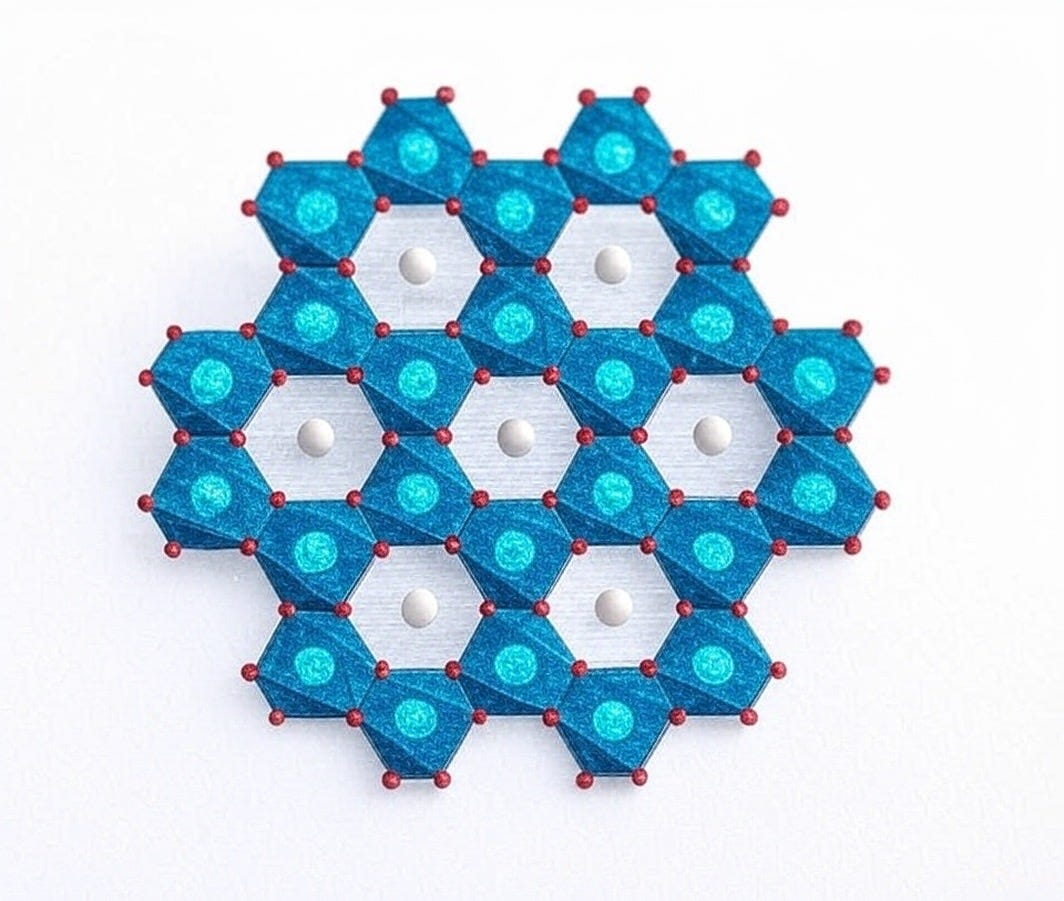
Lithium-aluminum layered double hydroxides (LiAl-LDHs) are aluminum-based materials used in adsorption-type Direct Lithium Extraction (DLE) to recover lithium from brine. These materials feature a disordered, layered structure with a chemical composition of LiX·2Al(OH)₃·nH₂O, where Li represents lithium, X is an anion (commonly chloride, Cl⁻), 2Al(OH)₃ forms the aluminum hydroxide framework, and nH₂O accounts for water molecules within the layers. This structure enables LiAl-LDHs to selectively capture lithium ions, establishing them as an effective method for lithium production.
Historical Context
Adsorption DLE began in the 1970s with DOW Chemical Company’s work in Smackover, Arkansas. DOW developed anion exchange resins paired with microcrystalline LiX·2Al(OH)₃ (where X is a halogen), as detailed in their 1979 patent, to selectively extract lithium from brines. This led to the development of Lithium Aluminum Layered Double Hydroxides (LiAl-LDHs), establishing a method for lithium recovery that shaped future DLE technologies.
DOW’s work directly influenced International Battery Metals Ltd. (IBAT), founded by Dr. John Burba, a key figure in DOW’s lithium research. IBAT’s DLE technology builds on this foundation, using a lithium aluminate-intercalate matrix sorbent, a derivative of DOW’s early work, to achieve high-efficiency lithium extraction.
DOW’s legacy also impacted companies like Standard Lithium and Anson Resources. Both use Koch Technology Solutions’ Li-Pro technology, which is heavily influenced by DOW’s original work—Standard Lithium in the Smackover region and Anson Resources in Utah—to refine lithium extraction with modern engineering. Albemarle has also explored LDH-based approaches for brine processing, further reflecting DOW’s influence on the industry.
Side Note: DLE platforms are customized for each location, using a series of steps to convert source brine into purified lithium chloride (LiCl): pretreatment (to remove solids and organic compounds for process compatibility), ion exchange softening (to eliminate interfering ions and prevent scaling), reverse osmosis (RO) concentration (to increase lithium concentration by removing water), polishing and final solid creation (which can involve evaporation and crystallization to remove excess water and form lithium chloride crystals), and solid-liquid separation (to isolate purified lithium chloride from residual impurities).
This report focuses on the LiAl-LDH sorbent used in adsorption-based DLE systems, which performs the actual lithium extraction.
Mechanism of Lithium Adsorption
The adsorption process involves lithium ions (Li⁺) attaching to a sorbent material as brine flows through. In modern platforms, this mechanism is refined for continuous operation and efficiency:
Lithium Capture: Lithium-aluminum layered double hydroxide (LiAl-LDH) is a layered material with a framework of aluminum and oxygen atoms, forming [LiAl₂(OH)₆]⁺ sheets. Within these sheets are octahedral vacancies—six-sided pockets lined by six oxygen atoms—where lithium ions (Li⁺) fit perfectly. With a small ionic radius of 76 picometers (pm) and a single positive charge, Li⁺ slots into these sites like a key in a lock.